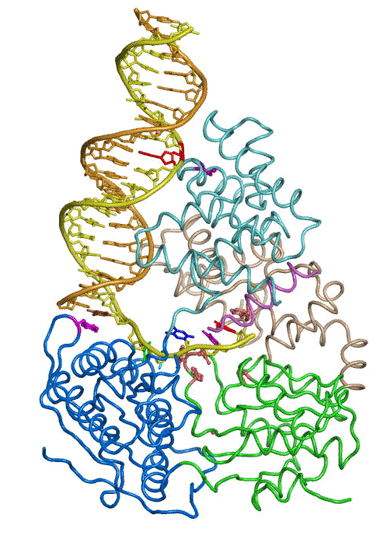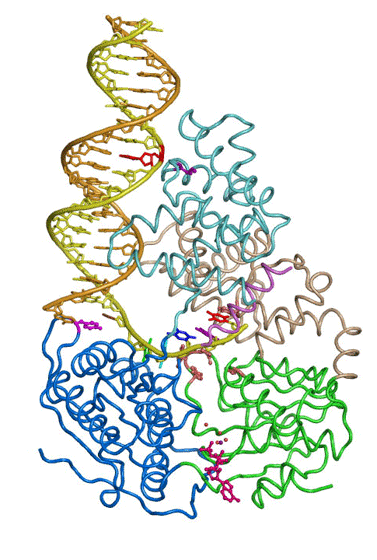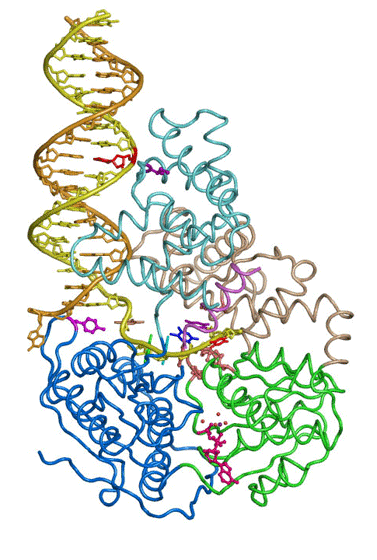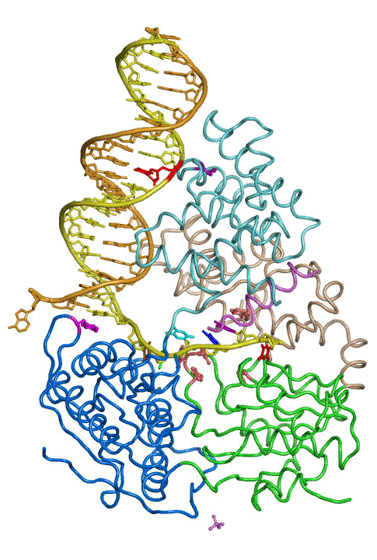
Genomic DNA is susceptible to a variety of mutagenic processes. Our group studies mismatch repair, which corrects replication errors, translesion DNA synthesis, which completes DNA replication when normal polymerases are stalled by damaged bases, and V(D)J recombination, which uses sequence-specific nuclease RAG1/2 and Non-Homologous End Joining (NHEJ) to assemble millions of antigen receptor genes for lymphocyte maturation.
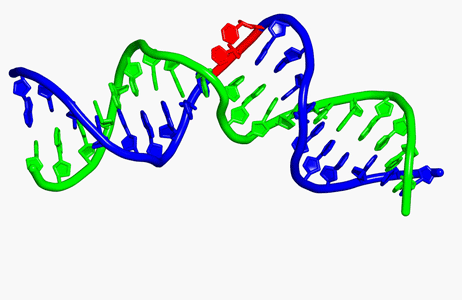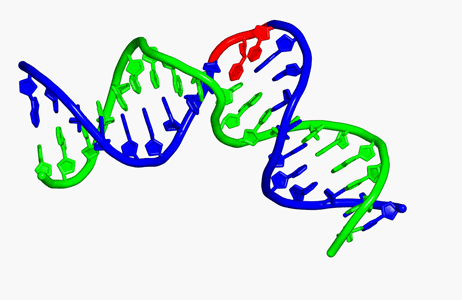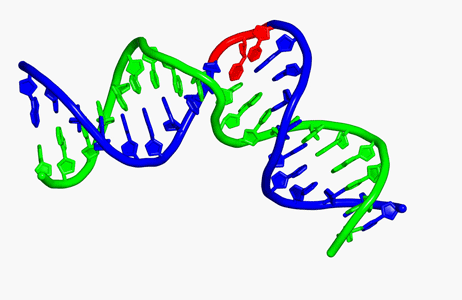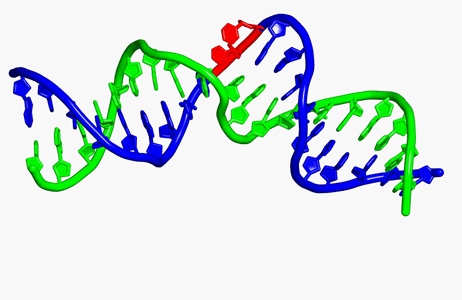
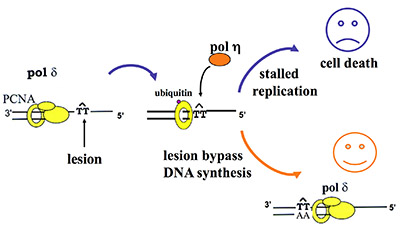
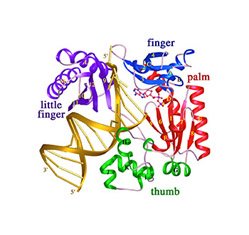
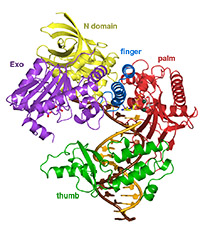
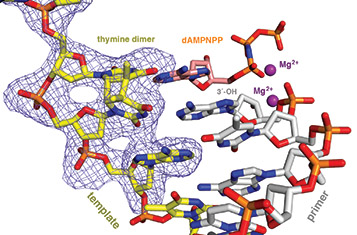
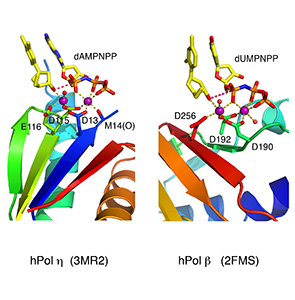
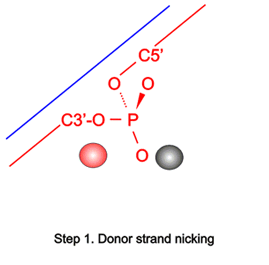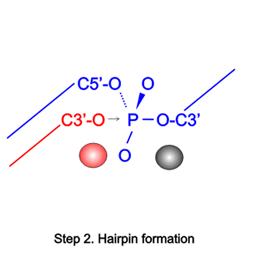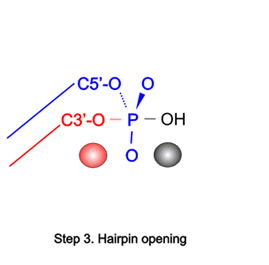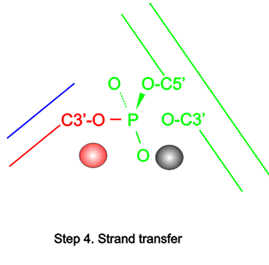
Translesion DNA synthesis (TLS) is most often carried out by the specialized Y-family DNA polymerases, which are characterized by an enlarged and solvent-exposed active site and the absence of an intrinsic proofreading exonuclease activity. However, some A- and B-family DNA polymerases also perform TLS although they share the highly conserved active site with high-fidelity replicative polymerases and occasionally even the proofreading function. TLS has implications in both cancer avoidance and resistance to chemotherapy.
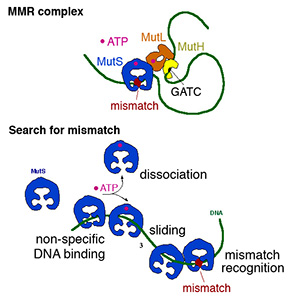
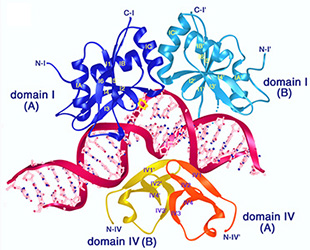
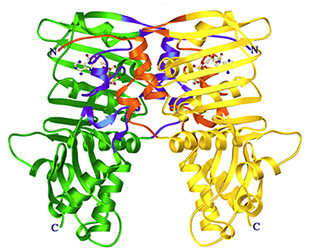
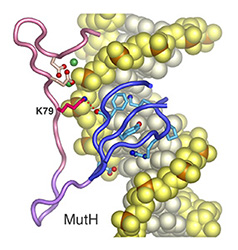
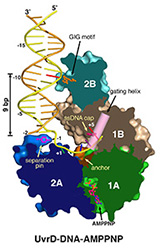
Misincorporation and slippage during DNA replication occur at a frequency of 10-8to 10-6 and result in mispaired or unpaired bases. Failure to correct replication errors by mismatch repair leads to genome instability and Lynch Syndrome (predisposition of hereditary cancers) in humans. MisMatch Repair (MMR) is initiated by two highly conserved ATPases MutS and MutL. In E. coli, MMR also requires MutH endonuclease and UvrD helicase to remove a mismatched base.
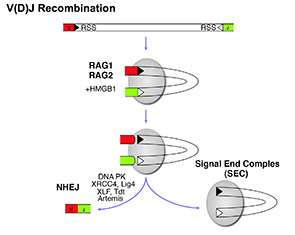
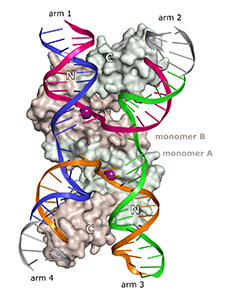
The large repertoire of immunoglobulins and T cell receptors is generated in many vertebrates by combinatorial rearrangement of an extensive array of variable (V), diversity (D), and joining (J) gene segments that are joined to encode the variable domains of the protein chains. RAG1 and RAG2 proteins are highly conserved among jawed vertebrates and initiate V(D)J recombination by cleaving Recombination Signal Sequences (RSS) flanking the V, D and J gene segments. RAG1/2 expression and activity are strictly regulated, and aberrant recombination leads to severe combined immune deficiency (SCID) and lymphoma. Cleaved DNA segments are joined by NHEJ process.
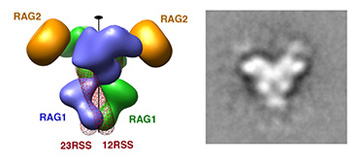
SEC: Signal Enc Complex
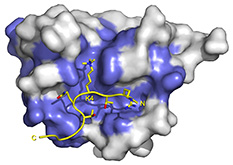
PHD: RAG2-PHD
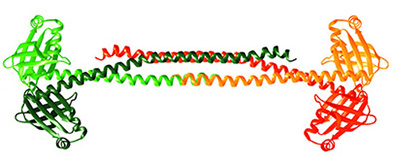
Xrcc4: Xrcc4 tetramer
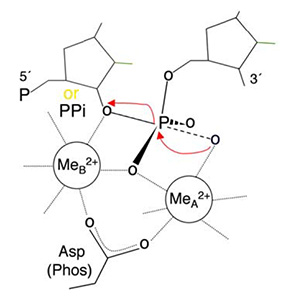
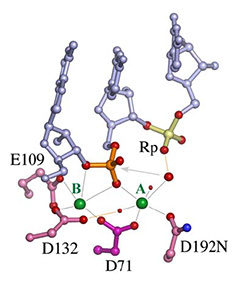
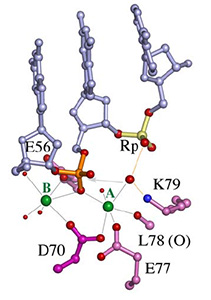
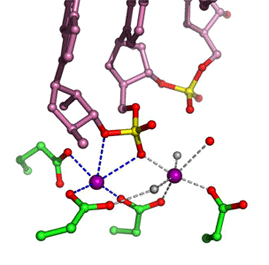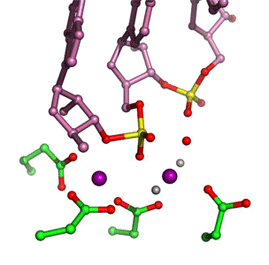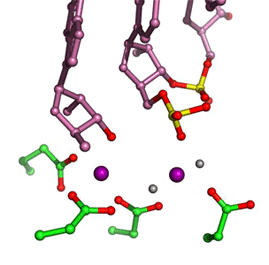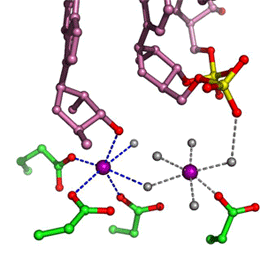
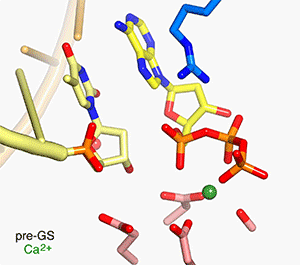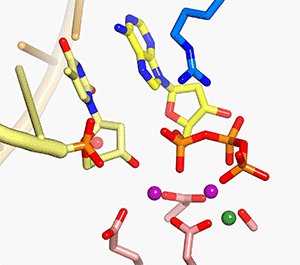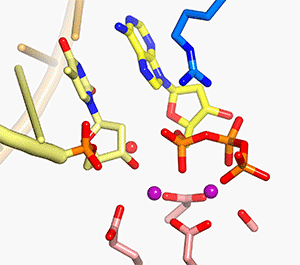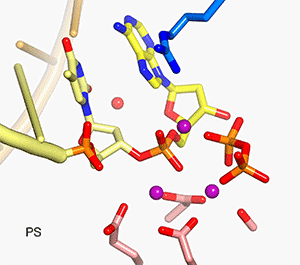
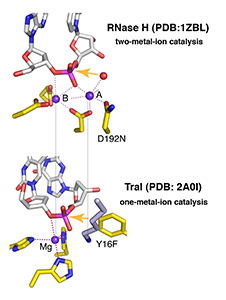
In all DNA and RNA polymerases and many nucleases and transposases, two Mg2+ ions are jointly coordinated by the nucleic acid substrate and catalytic carboxylates of the enzyme. Based on the exquisite sensitivity of Mg2+ ions to the ligand geometry and electrostatic environment, we propose that two-metal ion catalysis greatly enhances substrate recognition and catalytic specificity. Other nucleic acid enzymes, e.g. the β-β-α-Me and HUH nucleases, depend on a single metal ion for catalysis. We find that the one- and two-metal ion-dependent enzymes share a conserved metal ion-binding site.
SEC: Signal Enc Complex
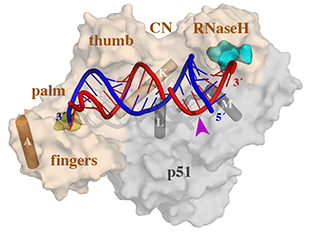
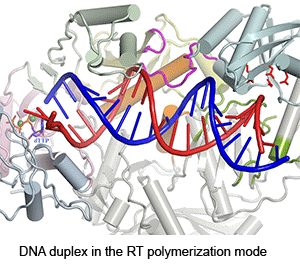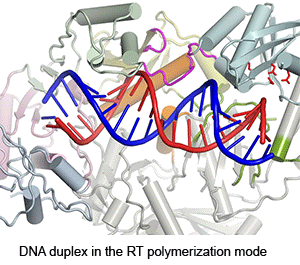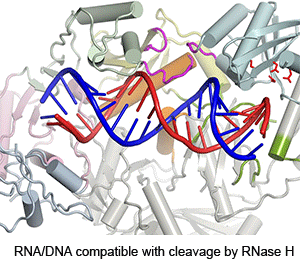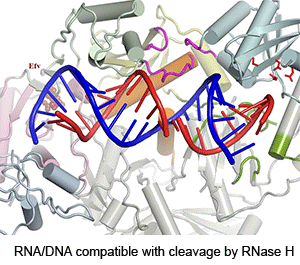
Four enzymatic activities are encoded by HIV. Three of them, DNA polymerase, RNase H and integrase, use two-metal-ion dependent catalysis, and RNase H and integrase share a conserved catalytic mechanism. The polymerase and integrase catalytic centers have been successfully used as drug targets for anti-HIV treatment. Supported by NIH IATAP, we have been studying HIV RNase H and integrase as potential anti-HIV drug targets.